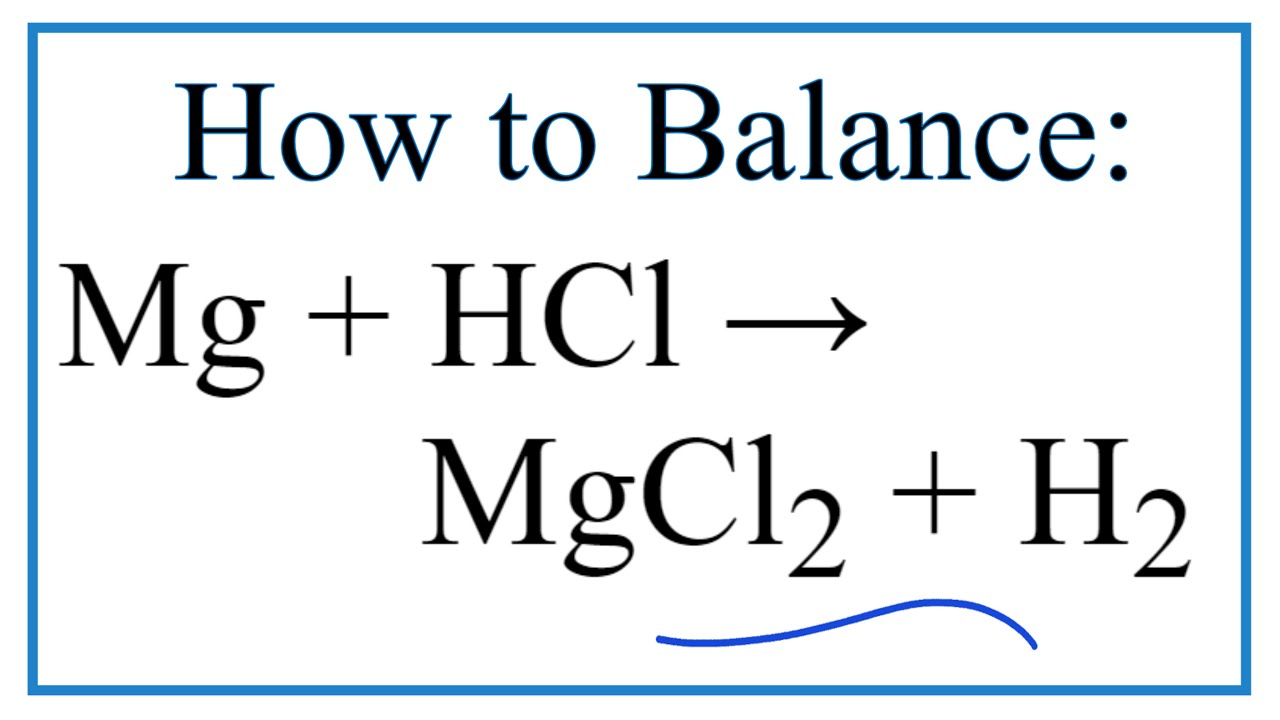
Does Hydrochloric Acid Produce Carbon Dioxide . The h 2 co 3 is a product of. reactions of acids with carbonates and hydrogen carbonates. the balanced equation shows that one mole of calcium carbonate reacts with two moles of hydrochloric acid to produce one mole. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: the carbon dioxide causes bubbling during the reaction, which is observed as fizzing. These reactions produce salt, water and carbon. like all metal carbonates, calcium carbonate reacts with acidic solutions to produce carbon dioxide gas. Carbonic acid (h 2 co 3) is a weak acid found in carbonated water. It is this reaction that. acids react with metal carbonates and hydrogencarbonates in the same way. It can be detected by passing the gas. so, for example, if you add dilute hydrochloric acid to solid sodium hydrogencarbonate, it will react giving off colourless carbon dioxide gas and.
from www.tessshebaylo.com
the carbon dioxide causes bubbling during the reaction, which is observed as fizzing. Carbonic acid (h 2 co 3) is a weak acid found in carbonated water. reactions of acids with carbonates and hydrogen carbonates. It can be detected by passing the gas. It is this reaction that. The h 2 co 3 is a product of. the balanced equation shows that one mole of calcium carbonate reacts with two moles of hydrochloric acid to produce one mole. These reactions produce salt, water and carbon. so, for example, if you add dilute hydrochloric acid to solid sodium hydrogencarbonate, it will react giving off colourless carbon dioxide gas and. acids react with metal carbonates and hydrogencarbonates in the same way.
Chemical Equation For The Acid Ionization Of Hydrochloric Hcl In Water
Does Hydrochloric Acid Produce Carbon Dioxide It can be detected by passing the gas. reactions of acids with carbonates and hydrogen carbonates. like all metal carbonates, calcium carbonate reacts with acidic solutions to produce carbon dioxide gas. so, for example, if you add dilute hydrochloric acid to solid sodium hydrogencarbonate, it will react giving off colourless carbon dioxide gas and. the balanced equation shows that one mole of calcium carbonate reacts with two moles of hydrochloric acid to produce one mole. The h 2 co 3 is a product of. These reactions produce salt, water and carbon. the carbon dioxide causes bubbling during the reaction, which is observed as fizzing. It can be detected by passing the gas. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: acids react with metal carbonates and hydrogencarbonates in the same way. Carbonic acid (h 2 co 3) is a weak acid found in carbonated water. It is this reaction that.
From www.tessshebaylo.com
Balanced Equation For Baking Soda And Hydrochloric Acid Tessshebaylo Does Hydrochloric Acid Produce Carbon Dioxide reactions of acids with carbonates and hydrogen carbonates. the carbon dioxide causes bubbling during the reaction, which is observed as fizzing. so, for example, if you add dilute hydrochloric acid to solid sodium hydrogencarbonate, it will react giving off colourless carbon dioxide gas and. It is this reaction that. These reactions produce salt, water and carbon. Web. Does Hydrochloric Acid Produce Carbon Dioxide.
From www.alamy.com
Diagram of the laboratory preparation of carbon dioxide from Does Hydrochloric Acid Produce Carbon Dioxide It can be detected by passing the gas. These reactions produce salt, water and carbon. It is this reaction that. acids react with metal carbonates and hydrogencarbonates in the same way. reactions of acids with carbonates and hydrogen carbonates. like all metal carbonates, calcium carbonate reacts with acidic solutions to produce carbon dioxide gas. Carbonic acid (h. Does Hydrochloric Acid Produce Carbon Dioxide.
From www.vrogue.co
An Aqueous Solution Of Sodium Hydroxide Reacts Comple vrogue.co Does Hydrochloric Acid Produce Carbon Dioxide It is this reaction that. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: so, for example, if you add dilute hydrochloric acid to solid sodium hydrogencarbonate, it will react giving off colourless carbon dioxide gas and. acids react with metal carbonates and hydrogencarbonates in the same way. reactions of acids. Does Hydrochloric Acid Produce Carbon Dioxide.
From www.britannica.com
Acid rain Chemistry, Pollutants, Effects Britannica Does Hydrochloric Acid Produce Carbon Dioxide The h 2 co 3 is a product of. These reactions produce salt, water and carbon. the balanced equation shows that one mole of calcium carbonate reacts with two moles of hydrochloric acid to produce one mole. like all metal carbonates, calcium carbonate reacts with acidic solutions to produce carbon dioxide gas. the carbon dioxide causes bubbling. Does Hydrochloric Acid Produce Carbon Dioxide.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Does Hydrochloric Acid Produce Carbon Dioxide so, for example, if you add dilute hydrochloric acid to solid sodium hydrogencarbonate, it will react giving off colourless carbon dioxide gas and. reactions of acids with carbonates and hydrogen carbonates. the carbon dioxide causes bubbling during the reaction, which is observed as fizzing. The h 2 co 3 is a product of. acids react with. Does Hydrochloric Acid Produce Carbon Dioxide.
From www.teachoo.com
Case Based MCQ Sanjana while preparing cake used Science Class 10 Does Hydrochloric Acid Produce Carbon Dioxide the carbon dioxide causes bubbling during the reaction, which is observed as fizzing. These reactions produce salt, water and carbon. acids react with metal carbonates and hydrogencarbonates in the same way. The h 2 co 3 is a product of. It is this reaction that. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide,. Does Hydrochloric Acid Produce Carbon Dioxide.
From basdemax.netlify.app
28++ Hydrochloric Acid And Sodium Hydroxide Balanced Equation Basdemax Does Hydrochloric Acid Produce Carbon Dioxide Carbonic acid (h 2 co 3) is a weak acid found in carbonated water. the balanced equation shows that one mole of calcium carbonate reacts with two moles of hydrochloric acid to produce one mole. It can be detected by passing the gas. like all metal carbonates, calcium carbonate reacts with acidic solutions to produce carbon dioxide gas.. Does Hydrochloric Acid Produce Carbon Dioxide.
From www.youtube.com
How to Balance CaCO3 + HCl = CaCl2 + CO2 + H2O (calcium carbonate Does Hydrochloric Acid Produce Carbon Dioxide the balanced equation shows that one mole of calcium carbonate reacts with two moles of hydrochloric acid to produce one mole. It can be detected by passing the gas. The h 2 co 3 is a product of. Carbonic acid (h 2 co 3) is a weak acid found in carbonated water. reactions of acids with carbonates and. Does Hydrochloric Acid Produce Carbon Dioxide.
From www.shalom-education.com
Testing for Carbonate Ions GCSE Chemistry Revision Does Hydrochloric Acid Produce Carbon Dioxide The h 2 co 3 is a product of. These reactions produce salt, water and carbon. like all metal carbonates, calcium carbonate reacts with acidic solutions to produce carbon dioxide gas. reactions of acids with carbonates and hydrogen carbonates. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: Carbonic acid (h 2. Does Hydrochloric Acid Produce Carbon Dioxide.
From pediaa.com
Difference Between Carbon Dioxide and Carbon Monoxide Definition Does Hydrochloric Acid Produce Carbon Dioxide like all metal carbonates, calcium carbonate reacts with acidic solutions to produce carbon dioxide gas. reactions of acids with carbonates and hydrogen carbonates. The h 2 co 3 is a product of. Carbonic acid (h 2 co 3) is a weak acid found in carbonated water. the balanced equation shows that one mole of calcium carbonate reacts. Does Hydrochloric Acid Produce Carbon Dioxide.
From www.slideshare.net
Chemical Reactions Does Hydrochloric Acid Produce Carbon Dioxide the balanced equation shows that one mole of calcium carbonate reacts with two moles of hydrochloric acid to produce one mole. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: Carbonic acid (h 2 co 3) is a weak acid found in carbonated water. These reactions produce salt, water and carbon. It can. Does Hydrochloric Acid Produce Carbon Dioxide.
From www.alamy.com
calcium carbonate reacts with dilute hydrochloric acid to produce Does Hydrochloric Acid Produce Carbon Dioxide Carbonic acid (h 2 co 3) is a weak acid found in carbonated water. It can be detected by passing the gas. the balanced equation shows that one mole of calcium carbonate reacts with two moles of hydrochloric acid to produce one mole. The h 2 co 3 is a product of. It is this reaction that. reactions. Does Hydrochloric Acid Produce Carbon Dioxide.
From www.coursehero.com
[Solved] CONCLUSIONS What is the gas formed in the hydrochloric acid Does Hydrochloric Acid Produce Carbon Dioxide so, for example, if you add dilute hydrochloric acid to solid sodium hydrogencarbonate, it will react giving off colourless carbon dioxide gas and. like all metal carbonates, calcium carbonate reacts with acidic solutions to produce carbon dioxide gas. The h 2 co 3 is a product of. acids react with metal carbonates and hydrogencarbonates in the same. Does Hydrochloric Acid Produce Carbon Dioxide.
From savanahminwells.blogspot.com
Calcium Carbonate Reacts With Hydrochloric Acid SavanahminWells Does Hydrochloric Acid Produce Carbon Dioxide like all metal carbonates, calcium carbonate reacts with acidic solutions to produce carbon dioxide gas. the carbon dioxide causes bubbling during the reaction, which is observed as fizzing. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: so, for example, if you add dilute hydrochloric acid to solid sodium hydrogencarbonate, it. Does Hydrochloric Acid Produce Carbon Dioxide.
From www.tessshebaylo.com
Chemical Equation For The Acid Ionization Of Hydrochloric Hcl In Water Does Hydrochloric Acid Produce Carbon Dioxide the carbon dioxide causes bubbling during the reaction, which is observed as fizzing. reactions of acids with carbonates and hydrogen carbonates. These reactions produce salt, water and carbon. It is this reaction that. acids react with metal carbonates and hydrogencarbonates in the same way. The h 2 co 3 is a product of. It can be detected. Does Hydrochloric Acid Produce Carbon Dioxide.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Does Hydrochloric Acid Produce Carbon Dioxide so, for example, if you add dilute hydrochloric acid to solid sodium hydrogencarbonate, it will react giving off colourless carbon dioxide gas and. reactions of acids with carbonates and hydrogen carbonates. acids react with metal carbonates and hydrogencarbonates in the same way. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water:. Does Hydrochloric Acid Produce Carbon Dioxide.
From www.alamy.com
calcium carbonate reacts with dilute hydrochloric acid to produce Does Hydrochloric Acid Produce Carbon Dioxide These reactions produce salt, water and carbon. It can be detected by passing the gas. like all metal carbonates, calcium carbonate reacts with acidic solutions to produce carbon dioxide gas. It is this reaction that. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: the carbon dioxide causes bubbling during the reaction,. Does Hydrochloric Acid Produce Carbon Dioxide.
From www.slideserve.com
PPT IGCSE Chemistry PowerPoint Presentation ID5408111 Does Hydrochloric Acid Produce Carbon Dioxide It can be detected by passing the gas. reactions of acids with carbonates and hydrogen carbonates. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: the balanced equation shows that one mole of calcium carbonate reacts with two moles of hydrochloric acid to produce one mole. The h 2 co 3 is. Does Hydrochloric Acid Produce Carbon Dioxide.